
Cholinesterase Monitoring of Agricultural Pesticide Applicators
To achieve its illness prevention goal, the medical supervision program is structured in such a manner that workers receive a pre-exposure cholinesterase baseline blood test that measures both the red blood cell (RBC) cholinesterase and plasma cholinesterase enzymes.
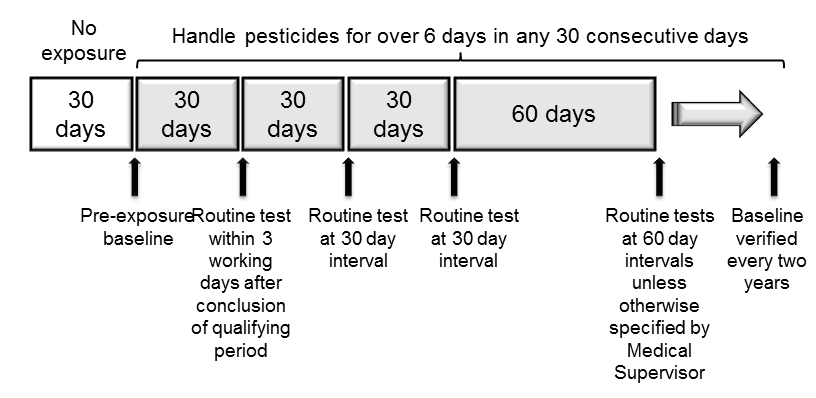
For workers who regularly handle these more highly toxic organophosphate and carbamate pesticides, periodic test results are compared against each individual’s baseline to determine if the employee should be removed from continued pesticide exposure.
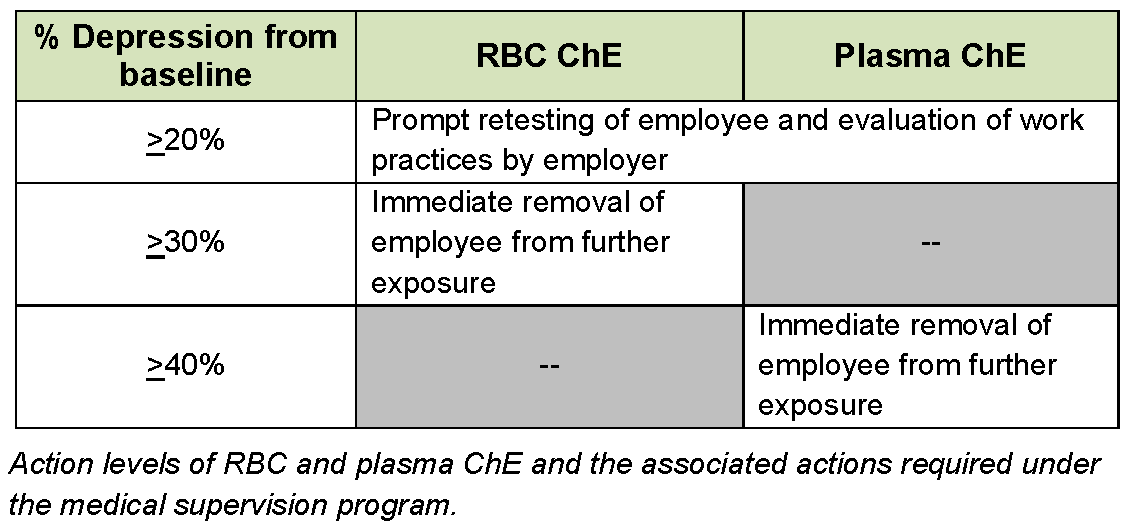
Assuring that these steps occur requires the excellent communication, cooperation, and regulatory compliance among a number of participating entities.
The primary participants of the overall medical supervision program include:
- Employee: Any individual who carries out the tasks of mixing, loading or applying organophosphate and carbamate (cholinesterase-inhibiting) pesticides. This includes the staff of pesticide service companies, individual farmers who apply pesticides to their own fields, and any employees of individual farmers, farm managers, independent contractors, etc.
- Employer: The management of pesticide services businesses, farmers and other agricultural personnel who contract to have crops or fields treated with pesticides. As of January 1, 2017, employers are required to contract with a medical supervisor that is registered with OEHHA.
- Medical Supervisor: A physician who has a contract with an employer to provide occupational health evaluation and illness prevention to an employee. Effective January 1, 2017, all Medical Supervisors are required to register with OEHHA.
- Clinical Chemistry Laboratory: The entity that evaluates the cholinesterase status of blood samples from an employee.
- County Agricultural Commissioner: The office charged with administering the medical supervision program.
The schematic structure of this program is shown in the figure below (Click on image to enlarge):
The relationships between these elements are:
Employees and Employer:
- Employer provides medical supervision program for employees.
- Employer provides a safe working environment for employees.
- Employees handle pesticides in safe manner to reduce chances of exposure.
Employers and Medical Supervisor:
- Employers have written agreement with licensed physician(s) to provide occupational health evaluation and illness prevention, including cholinesterase monitoring.
- Employers agree to abide by recommendations of physician regarding working conditions for employees.
- Medical supervisor registers with OEHHA and provides workplace recommendation and employees’ cholinesterase testing schedules to employer.
Medical Supervisor and Employees:
- Employees abide by recommendations of medical supervisor regarding work hours, exposure reduction or avoidance.
- Medical supervisor is current on employee cholinesterase levels.
- Medical supervisor establishes pre-exposure baseline cholinesterase values for employee.
Employers and County Agricultural Commissioner:
- Employers provide copies of Medical Supervision contracts to County Agricultural Commissioner.
- County Agricultural Commissioner keeps employers updated on Medical Supervision program changes or regulations promulgated by the State Department of Pesticide Regulation.
Employees and Laboratory:
- Employees should be consistent in the laboratory s/he uses for blood draw.
- Laboratory collects blood samples for plasma and RBC ChE measurements.
Laboratory and Medical Supervisor:
- Medical Supervisor should be consistent in the labs s/he refers employees to and should use a California Department of Public Health-approved laboratory.
- Laboratories should provide timely results to medical supervisor flagging any out of range results or other anomalies that might affect the employee’s health (quality control issues; blood draw problems).
Laboratory and Department of Pesticide Regulation:
- Labs must now report all pesticide-related cholinesterase test results electronically to the California Department of Pesticide Regulation.
DPR and OEHHA and DPH:
- DPR shares the cholinesterase test results electronically with OEHHA and DPH.
- DPR, OEHHA and DPH use the cholinesterase data to evaluate the effectiveness of the Medical Supervision Program.
