Acidification of Coastal Waters

Credit: Jeremy Bishop
Carbon dioxide, the most important driver of climate change, is continuously exchanged between land, the atmosphere, and the ocean through physical, chemical, and biological processes. The oceans absorb about one-third of the carbon dioxide emitted by human activities. This absorption has reduced levels of the gas in the atmosphere and slowed the rate of global warming. The large amounts absorbed by the oceans are changing its chemistry, including acidifying seawater. These changes can have major effects on marine life. For more information, download the Acidification of Coastal Waters chapter.
The animation below shows changes in surface ocean chemistry from 1800 through 2020. The change from green to red illustrates decreasing levels of “aragonite saturation.” Aragonite is the most soluble form of calcium carbonate, which is needed by marine organisms to form shells or skeletons. Lower aragonite saturation values indicate that it will be more challenging for organisms to make shells. The inset box shows increasing levels of carbon dioxide in the atmosphere.
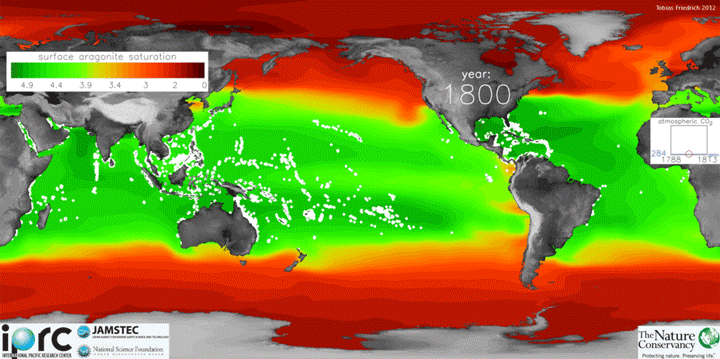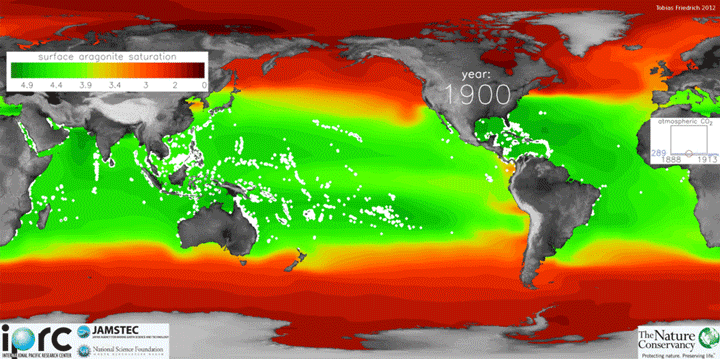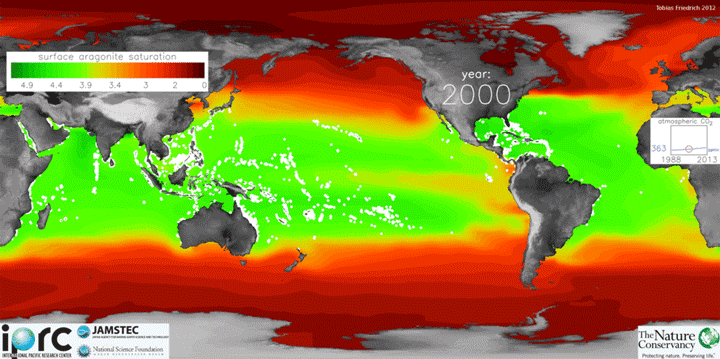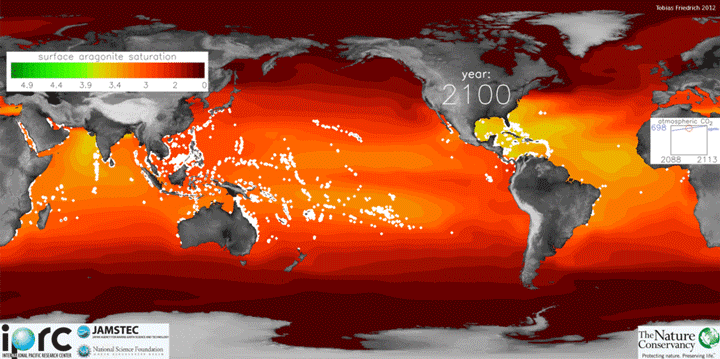
Credit: Tobias Friedrich/University of Hawaii
What does the indicator show?
Carbon dioxide (CO2) levels off the coasts of California and Hawaii
This graph shows the levels of CO2 in seawater (measured as pCO2, in microatmospheres) off of Hawaii and 140 miles and 20 miles off of Point Conception, California (CCE1 and CCE2, respectively).
- From 1988 to 2024, carbon dioxide levels in seawater off Hawaii have increased steadily (at the rate of about 2 microatmospheres per year). The increases have been accompanied by increasing acidity (measured as pH levels, not shown on graph). Monitoring off Hawaii provides the longest-running measurements of ocean acidity in the North Pacific Ocean.
- Continuous monitoring off the California coast started around 2008 off Point Conception in Central California. Over the past decade, carbon dioxide levels in seawater measured 140 miles off the coast have increased and largely track the measurements off Hawaii. Closer to shore, carbon dioxide levels are also increasing, but with greater fluctuations influenced by upwelling, a seasonal wind-driven process (discussed further in “What factors influence this indicator?”).
Why is this indicator important?
- Changes in ocean chemistry resulting from the absorption of carbon dioxide from the atmosphere have significant ecological consequences. Several biological processes in marine organisms are sensitive to changes in seawater chemistry.
- The best-documented and most widely observed effects involve the disruption of shell formation or the dissolution of shells under acidic conditions. Oysters, mussels, coral, and other organisms that build shells or skeletons are especially vulnerable to the effects of ocean acidification.
- Fish are sensitive to even small changes in water chemistry.
- Effects on prey can be amplified up the food chain, leading to wide-ranging disruptions to marine ecosystems.
- Marine ecosystems are threatened by the combined effects of ocean acidification, warming waters, and reduced levels of dissolved oxygen.
- California’s coastal economies, which rely on commercial and recreational seafood industries, could suffer severe losses as marine ecosystems become impacted by ocean acidification
- Tracking ocean acidification provides a better understanding of the distribution of carbon emissions into the atmosphere, land, and oceans. Information on the capacity of lands and oceans to store carbon (that is, to serve as “sinks”) is useful for developing strategies to slow the buildup of carbon dioxide in the atmosphere and for formulating climate policies.
Many ecologically and economically important marine species are threatened by ocean acidification, including
the sea butterfly (Limacina helicina), krill, Dungeness crab, and clams (pictured, from left to right).

Credit: NOAA
What factors influence the indicator?
- Increased carbon dioxide emissions from human activities is the main driver for the global decline in ocean water pH over the last 40 years. Differences in concentration in air compared to sea water drive the air-ocean carbon dioxide exchange.
- The amount of carbon in ocean water is influenced by biological reactions such as photosynthesis, respiration, and dissolution of calcium carbonate. Coastal waters can be affected by local factors, including organic matter and nutrient discharges from land and processes in the underlying sediments.
- Along the California coast, a wind-driven process called “upwelling” brings deeper, carbon dioxide-rich waters to the surface. Increasing ocean acidification adds to the naturally high levels of carbon dioxide in the upwelled waters.
- In addition to seasonal patterns, longer term changes associated with El Niño and the Pacific Decadal Oscillation can alter the ability of oceanic waters to serve as either a sink or a source of carbon dioxide.
Additional resources:
- National Oceanic and Atmospheric Administration (NOAA), Pacific Marine Environmental Laboratory Carbon Program, Ocean Acidification: The Other Carbon Dioxide
- National Oceanic and Atmospheric Administration (NOAA), National Centers for Environmental Information, Global Coastal Program Data
- Ocean Protection Council, Ocean Acidification and Hypoxia Program
- University of Hawaii at Manoa, Hawaii Ocean Time-series (HOT)
