Background for the December 17, 2002 Carcinogen Identification Committee Meeting Agenda Item: Consideration of Request to Review Statin Drugs as a Group for Subsequent Listing
The Office of Environmental Health Hazard Assessment (OEHHA) received a request from Mr. Gary Roberts on April 5, 2002 that the statin drugs all be reviewed for possible Proposition 65 listing by the Carcinogen Identification Committee (CIC) at the same time.
The statins are a class of lipid-lowering drugs. All statin drugs inhibit the enzyme 3-hydroxy-3-methylgluatryl-coenzymeA (HMG-CoA) reductase. HMG-CoA reductase catalyzes the conversion of hydroxymethylglutaryl-CoA to mevalonic acid, an early rate-limiting step in cholesterol biosynthesis. In clinical studies, statins reduce total cholesterol, LDL cholesterol, apolipoprotein B and triglyceride levels. Statins increase HDL levels. The normal treatment regimen for these drugs involves daily exposure over a period of many years.
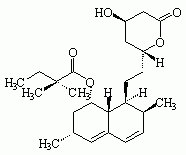
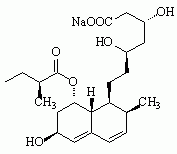
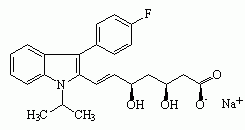
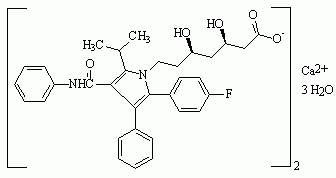
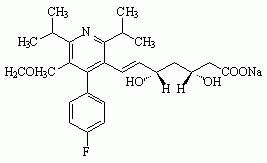
Six drugs in the "statin" class have been marketed for their hypolipidemic effects (see Figure 1): atorvastatin calcium (LIPITOR), cerivastatin sodium (BAYCOL), fluvastatin sodium (LESCOL), lovastatin (MEVACOR), pravastatin sodium (PRAVACHOL), and simvastatin (ZOCOR). Cerivastatin sodium was withdrawn from the U.S. market by the manufacturer in August 2001 because of reports of fatal rhabdomyolysis (disintegration of muscle tissue). All statins have been associated with rhabdomylosis, but severe cases have been reported more frequently with cerivastatin sodium.
Figure 1. Structures of statin drugs
LovastatinSimvastatin
Pravastatin sodium
Fluvastatin sodium
Atorvastatin calcium
Cerivastatin sodium
The prioritization process usually involves random selection of chemicals being tracked (OEHHA, 1997). Currently, only one of the six statin drugs, lovastatin, has been prioritized and randomly selected (in February 1999) for consideration by the CIC for listing under Proposition 65. The other statin drugs are being tracked as a group, but have not been randomly selected for prioritization as to carcinogenicity concern. As stated in the prioritization process document, "(u)nder exceptional circumstances, the process may be abbreviated to allow OEHHA to respond to specific public health needs. Following consultation with the Committee Chair, the Director of OEHHA may request that a chemical be placed on the agenda of the next scheduled meeting." In consideration of Mr. Roberts' request and consistent with the prioritization process, the Director of OEHHA in consultation with the Chair of the CIC included on the agenda the request for discussion at the December 17, 2002 meeting.
As noted in the U.S. Food and Drug Administration's approved package inserts for these six statin drugs, long-term administration of the individual statins to experimental animals has been associated with the induction of tumors at various sites. Information on tumor induction is outlined in Table 1.
Table 1. Reported carcinogenicity of statin drugs, by tumor site
- Liver
- atorvastatin calcium (carcinoma, female mice; adenoma, male mice)
- cerivastatin sodium (carcinoma, male mice; adenoma, male and female mice)
- lovastatin (carcinoma, male and female mice; male rats)
- pravastatin sodium (carcinoma, male rats)
- simvastatin (carcinoma, mice and rats of both sexes)
- Thyroid
- simvastatin (adenoma, female rats)
- fluvastatin sodium (adenoma and carcinoma, male rats)
- Lung
- lovastatin (adenoma, female mice)
- simvastatin (adenoma, male and female mice)
- Forestomach
- lovastatin (papilloma, female mice)
- fluvastatin sodium (papilloma, male and female mice)
- Harderian gland
- simvastatin (adenoma, male and female mice)
- Muscle
- atorvastatin calcium (two rare tumors, rats)
Five of the statins induced tumors of the liver in rodents, and tumors of the thyroid, lung and forestomach have each been induced by one or more statins. The frequent concordance in tumor sites between rats and mice (Table 1) and structural (Figure 1), pharmacologic and toxicologic similarities suggest that a common mechanism of action is involved in at least some of the carcinogenic effects. The preparation of a cancer hazard identification document on one statin (e.g., lovastatin) would necessarily entail a review of the information available on the other statins. At the request of the CIC, OEHHA can prepare a single document that comprehensively presents the evidence of carcinogenicity for each of the statin drugs.
Link to Public Comments
Chemical Reference
- Lovastatin
- Pravastatin sodium
- Simvastatin
- Atorvastatin calcium
- Fluvastatin sodium
- Cerivastatin sodium
Related Notices
Footnotes and References
References
Office of Environmental Health Hazard Assessment (OEHHA, 1997). Procedure for Prioritizing Candidate Chemicals for Consideration Under Proposition 65 by the "State's Qualified Experts," Reproductive and Cancer Hazard Assessment Section, OEHHA, California Environmental Protection Agency, May 1997.
Links to:
CIC Identification Committee Roster
Governor's Press Release, August 22, 2002
Links to documents related to statin drugs request:
O'Donnell and Shaeffer request letter
OEHHA response letter
This Statin background document